How Quality Management Systems Define Reliability in Electronics Manufacturing
- What a QMS Means in the EMS Context
- Why Quality Systems Are Essential in EMS Manufacturing
- The Foundation: ISO 9001 in Electronics Manufacturing
- AS9100: The Aerospace and Defense Benchmark
- ISO 13485: Quality Systems for Medical Electronics
- IPC and UL Standards: The Workmanship Backbone
- How Certification Translates to Reliability
- The Path to Certification
- The Future of Quality in EMS
- Conclusion: Quality as a Core Competency
- Partner with Federal Electronics
In the Electronics Manufacturing Services (EMS) industry, product reliability isn’t negotiable—it’s mission-critical. From aerospace and defense to medical and industrial electronics, even the smallest solder joint or connector fault can compromise an entire system. Customers demand absolute precision, regulators require documented proof of quality, and Original Equipment Manufacturers (OEMs) expect partners who can consistently deliver on both.
Behind this consistency lies a robust Quality Management System (QMS)—the backbone of every high-performing EMS provider. When effectively implemented, a QMS ensures that each process, inspection, and supplier interaction functions as part of an integrated ecosystem built to prevent errors, enforce standards, and continuously improve outcomes.
What a QMS Means in the EMS Context
A Quality Management System is more than a set of policies—it’s the operational architecture that governs how electronic assemblies are built, tested, and verified.
Within an EMS environment, a QMS integrates engineering, production, supply-chain and quality assurance controls into one traceable framework that guarantees every printed circuit board assembly (PCBA), cable assembly and wire harness meets both customer and regulatory requirements.
An EMS-specific QMS typically includes:
- Documented quality procedures aligned with ISO 9001 or sector-specific standards like AS9100 and ISO 13485.
- Process control documentation such as traveler sheets, work instructions, and revision control logs.
- Workmanship criteria defined by IPC-A-610 (for PCBAs) and IPC/WHMA-A-620 (for cable and wire harness assemblies).
- Soldering and inspection standards per J-STD-001, ensuring consistent reliability at the component level.
- Testing and validation checkpoints including Automated Solder Paste Inspection, Automated Optical Inspection (AOI), Flying Probe, and functional testing.
- Traceability systems that link serial numbers, lot codes, and materials back to supplier data and operator records.
- Electrostatic Discharge (ESD) control protocols that safeguard sensitive electronic components during handling and assembly.
This integrated structure creates a closed-loop feedback system—where quality issues are not just corrected, but prevented from recurring.

Why Quality Systems Are Essential in EMS Manufacturing
In electronics manufacturing, a defect isn’t just an increase in processing cost—it’s a potential failure in the field. A robust QMS minimizes that risk through:
- Process uniformity: Every operator, station, and shift follows the same validated steps.
- Reduced rework and scrap: Nonconformances are detected early through in-process audits and automated testing.
- Supplier accountability: Component and material suppliers are qualified, monitored and periodically re-audited.
- Regulatory confidence: Certifications demonstrate adherence to international expectations for safety and reliability.
- Customer trust: OEMs partnering with certified EMS providers gain assurance that every assembly meets documented specifications.
For many EMS companies, achieving certification to AS9100 or ISO 13485 can open entire sectors of opportunity—defense programs, medical equipment manufacturing, and mission-critical aerospace applications—where quality assurance is a contractual requirement.
The Foundation: ISO 9001 in Electronics Manufacturing
ISO 9001 is the global baseline for quality management across many industries. In EMS, it establishes the systematic controls that make repeatable quality possible—risk-based thinking, corrective and preventive actions, and data-driven improvement.
Key benefits of ISO 9001 compliance for EMS providers include:
- Establishing documented workflows for critical elements such as component handling, Electrostatic Discharge (ESD) protection, and process verification.
- Continuous improvement cycles that track KPIs in an effort to reduce defect rates, and warranty claims.
- A standardized framework that simplifies expansion to AS9100 and ISO 13485 certifications.
In essence, ISO 9001 creates the foundation upon which more specialized standards are built.

AS9100: The Aerospace and Defense Benchmark
For EMS providers serving aerospace, American defense manufacturing, or space industries, AS9100 is the defining quality management standard. It extends ISO 9001 with rigorous requirements for traceability, risk management, and configuration control—essential in environments where failure is not an option.
AS9100 adds:
- Comprehensive risk-based planning and counterfeit-part prevention
- Formal configuration and design-change management
- Enhanced supplier oversight and verification systems
- Mandatory product safety and process validation controls
AS9100-certified EMS companies demonstrate that their PCBA, cable assembly, wiring harness and electronics manufacturing processes meet the highest levels of traceability and reliability demanded by prime contractors of military communications, navigation, and command and control systems.
ISO 13485: Quality Systems for Medical Electronics
In the medical sector, ISO 13485 governs the design and manufacture of electronic devices that affect patient safety.
EMS providers producing electronic assemblies for medical devices and life-sustaining equipment must adhere to strict process controls, documentation, and traceability standards—often integrating medical device testing and specialized validation procedures.
This certification ensures that medical electronics meet both regulatory and clinical performance requirements, supporting compliance with FDA and international regulations.
IPC and UL Standards: The Workmanship Backbone
While ISO and AS standards govern management systems, IPC and UL standards define how quality is physically built into the product.
- IPC-A-610 sets acceptance criteria for PCBA workmanship—covering topics such as solder joint integrity, component alignment, and cleanliness.
- J-STD-001 governs soldering materials and processes, ensuring electrical reliability under thermal and mechanical stress.
- IPC/WHMA-A-620 defines quality requirements for cable and wire harness assemblies, including crimping, splicing, and connector workmanship.
- UL 508A applies to industrial control panels, ensuring electrical safety and component compliance especially for industrial equipment and automation applications.
Together, these standards serve as the operational “how-to” of electronics manufacturing, ensuring every finished assembly meets the intended design, performance, and safety requirements.
How Certification Translates to Reliability
Certification is more than a logo—it’s a measurable commitment to excellence.
An EMS provider certified to ISO 9001, AS9100, or ISO 13485 must maintain documented systems, conduct periodic internal audits, and undergo external surveillance audits to validate compliance.
These frameworks yield tangible benefits:
- Defect reduction – Consistent processes prevent rework and improve first-pass yield.
- Predictable quality – Statistical process control (SPC) and corrective actions create repeatability.
- Traceable supply chains – Every component can be traced from vendor to finished product.
- Operational efficiency – Standardized workflows reduce downtime and waste.
- Customer assurance – Certification demonstrates accountability and transparency at every stage.
For OEMs and prime contractors selecting EMS partners, these credentials serve as proof that manufacturing reliability is engineered—not assumed.
The Path to Certification
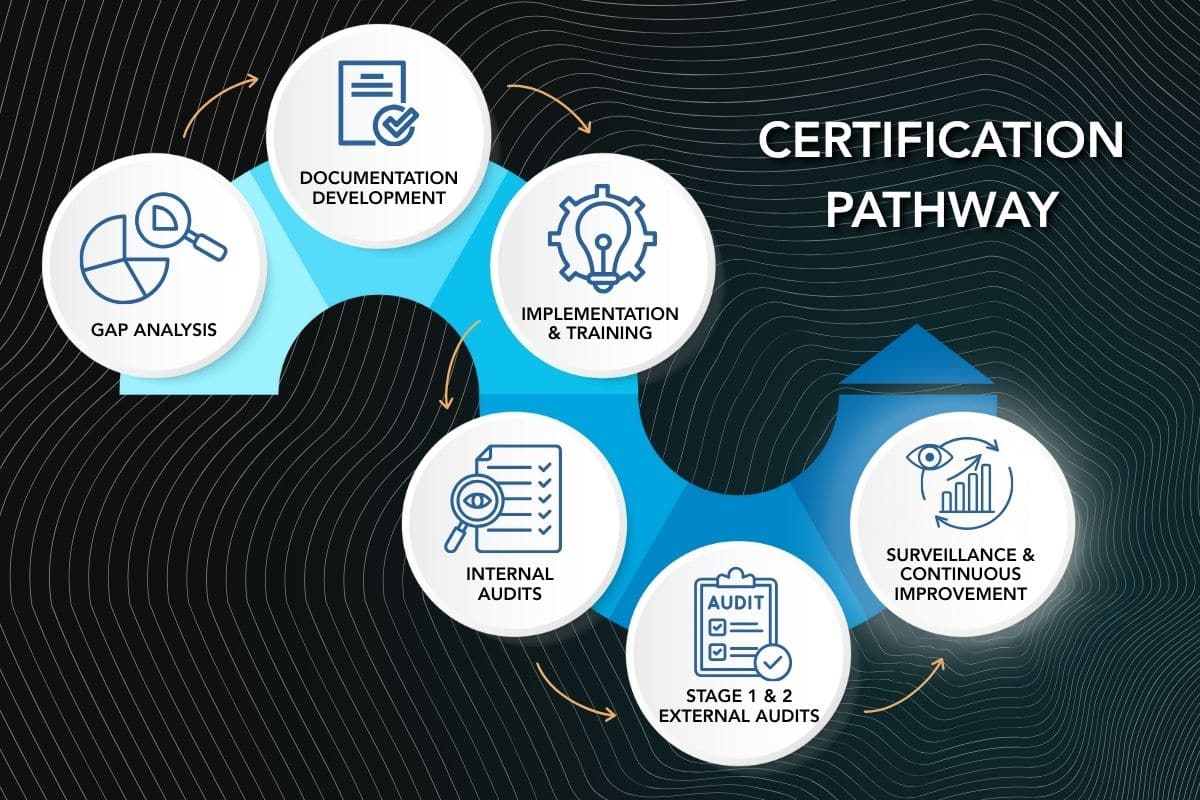
Achieving and maintaining certification involves a disciplined, multi-stage process:
- Gap Analysis – Benchmark existing processes against the target standard (ISO 9001, AS9100, or ISO 13485).
- Documentation Development – Create manuals, control plans, and training materials.
- Implementation & Training – Deploy standardized procedures across production lines.
- Internal Audits – Identify and correct nonconformities before third-party review.
- Stage 1 & 2 Certification Audits – Verify compliance and system effectiveness.
- Ongoing Surveillance Audits – Maintain continual improvement and prevent drift.
The goal isn’t a certificate—it’s a living system that continuously strengthens product quality and organizational reliability across highly regulated industries like medical, aerospace and defense.
The Future of Quality in EMS
As electronics manufacturing grows more complex, QMS frameworks are evolving.
Digital transformation now enables real-time quality dashboards, AI-based defect prediction, and automated traceability across multi-site operations.
Additionally, new layers such as NIST 800-171 and CMMC 2.0 are shaping cybersecurity expectations for defense-grade manufacturing, ensuring that data and design information are protected as rigorously as the products themselves.
The future EMS QMS will be as much about data integrity and security as it is about workmanship and inspection.
Conclusion: Quality as a Core Competency
In the EMS industry, quality management isn’t a department—it’s a culture.
Whether certified to AS9100, ISO 13485, ISO 9001, or compliant with IPC and UL standards, electronics manufacturers that prioritize QMS maturity turn reliability into reputation.
By embedding quality into every process EMS providers not only meet customer expectations but exceed them, ensuring that every Printed Circuit Board Assembly, cable assembly, wire harness, electronics assembly, and industrial control panel performs flawlessly in the field.
Partner with Federal Electronics
Contact us today to learn more about our solutions that are designed to drive your success.
